Barrett's esophagus
| Barrett's oesophagus | |
|---|---|
| Classification and external resources | |
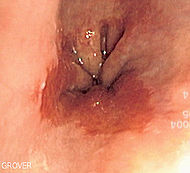 Endoscopic image of Barrett's oesophagus, which is the area of red mucosa projecting like a tongue. Biopsies showed intestinal metaplasia. |
|
| ICD-10 | K22.7 |
| ICD-9 | 530.85 |
| OMIM | 109350 |
| DiseasesDB | 1246 |
| MedlinePlus | 001143 |
| eMedicine | radio/73 |
| MeSH | D001471 |
Barrett's oesophagus (American English: esophagus) (sometimes called Barrett's syndrome, CELLO, columnar epithelium lined lower oesophagus) refers to an abnormal change (metaplasia) in the cells of the inferior portion of the oesophagus. A positive diagnosis generally requires observing specific macroscopic and microscopic changes. The normal squamous epithelium lining of the oesophagus is replaced by metaplastic columnar epithelium. Columnar epithelium refers to a cell type that is typically found in more distal parts of the gastrointestinal system. The medical significance of Barrett’s oesophagus is its strong association with oesophageal adenocarcinoma, a particularly lethal cancer.
The main cause of Barrett's oesophagus is thought to be an adaptation to chronic acid exposure from reflux oesophagitis.[1] In the last 40 years, the incidence of oesophageal adenocarcinoma has been increasing in the Western world. Barrett's oesophagus is found in 5-15% of patients who seek medical care for heartburn (gastroesophageal reflux disease, GERD), although a large subgroup of patients with Barrett's oesophagus do not have symptoms.[2] It is considered to be a premalignant condition because it is associated with an increased risk of oesophageal cancer (more specifically, adenocarcinoma) of about 5.5% per patient-year.[2][3] Diagnosis of Barrett's oesophagus requires endoscopy (more specifically, esophagogastroduodenoscopy, a procedure in which a small camera is inserted through the mouth to examine the oesophagus, stomach, and duodenum) and biopsy. The cells of Barrett's oesophagus, after biopsy, are classified into four general categories: non-dysplastic, low-grade dysplasia, high-grade dysplasia, and frank carcinoma. High grade dysplasia and frank carcinoma patients are generally advised to undergo surgical treatment. Non-dysplastic and low-grade patients are generally advised to undergo annual observation with endoscopy. In high-grade dysplasia, the risk of developing cancer might be at 10% per patient-year or greater.[2]
The condition is named after Norman Barrett (1903–1979) who described the condition in 1950.[4]
Contents |
Symptoms
The change from normal to premalignant cells that indicates Barrett's oesophagus does not cause any particular symptoms. Barrett's oesophagus, however, is associated with the following symptoms:
- frequent and longstanding heartburn
- trouble swallowing (dysphagia)
- vomiting blood
- pain under the breastbone where the oesophagus meets the stomach
- unintentional weight loss because eating is painful
The risk of developing Barrett's oesophagus is increased by central (vs. peripheral) obesity.[5] The exact mechanism is unclear. The difference in distribution of fat among men (more central) and women (more peripheral) may explain the increased risk in males.[6]
Mechanism
Barrett's oesophagus occurs due to chronic inflammation. The principal cause of the chronic inflammation is gastroesophageal reflux disease, GORD(USA: GERD). In this disease, acidic stomach contents cause damage to the cells of the lower oesophagus. Researchers are unable to predict which heartburn sufferers will develop Barrett's oesophagus. While there is no relationship between the severity of heartburn and the development of Barrett's oesophagus, there is a relationship between chronic heartburn and the development of Barrett's oesophagus. Sometimes people with Barrett's oesophagus will have no heartburn symptoms at all. In rare cases, damage to the oesophagus may be caused by swallowing a corrosive substance such as lye.
Diagnosis
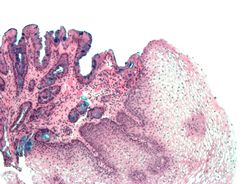
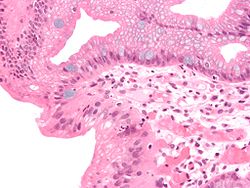
Both macroscopic (from endoscopy) and microscopic positive findings are required to make a diagnosis. Barrett's oesophagus is marked by the presence of columnar epithelia in the lower oesophagus, replacing the normal squamous cell epithelium—an example of metaplasia. The secretory columnar epithelium may be more able to withstand the erosive action of the gastric secretions; however, this metaplasia confers an increased risk of adenocarcinoma.[7]
The metaplastic columnar cells may be of two types: gastric (similar to those in the stomach, which is NOT technically Barrett's oesophagus) or colonic (similar to cells in the intestines). A biopsy of the affected area will often contain a mixture of the two. Colonic-type metaplasia is the type of metaplasia associated with risk of malignancy in genetically susceptible people.
The metaplasia of Barrett's oesophagus is grossly visible through a gastroscope, but biopsy specimens must be examined under a microscope to determine whether cells are gastric or colonic in nature. Colonic metaplasia is usually identified by finding goblet cells in the epithelium and is necessary for the true diagnosis of Barrett's.
There are many histologic mimics of Barrett's oesophagus (i.e. goblet cells occurring in the transitional epithelium of normal oesophageal submucosal gland ducts, "pseudogoblet cells" in which abundant foveolar (gastric) type mucin simulates the acid mucin true goblet cells). Assessment of relationship to submucosal glands and transitional-type epithelium with examination of multiple levels through the tissue may allow the pathologist to reliably distinguish between goblet cells of submucosal gland ducts and true Barrett's oesophagus (specialized columnar metaplasia). Use of the histochemical stain Alcian blue pH 2.5 is also frequently used to distinguish true intestinal-type mucins from their histologic mimics. Recently, immunohistochemical analysis with antibodies to CDX-2 (specific for mid and hindgut intestinal derivation) has also been utilized to identify true intestinal-type metaplastic cells. It has been shown that the protein AGR2 is elevated in Barrett's oesophagus,[8] and can be used as a biomarker for distinguishing Barrett's epithelium from normal oesophageal epithelium.[9]
After the initial diagnosis of Barrett's oesophagus is rendered, affected persons undergo annual surveillance to detect changes that indicate higher risk to progression to cancer: development of dysplasia. There is considerable variability in assessment for dysplasia among pathologists. Recently, gastroenterology and GI pathology societies have recommended that any diagnosis of high grade dysplasia in Barrett's be confirmed by at least two fellowship trained GI pathologists prior to definitive treatment for patients.
Management
Many professional medical societies propose endoscopic screening of patients with GERD and endoscopic surveillance of patients with Barrett's oesophagus, although little direct evidence supports this practice, which is common in many developed countries.[2] Treatment options for high-grade dysplasia include surgical removal of the oesophagus (esophagectomy) or endoscopic treatments such as endoscopic mucosal resection or ablation (destruction). Currently, there is no intervention that has been shown to prevent the development of Barrett's oesophagus or its progression to oesophageal cancer.[2]
The risk of malignancy is highest in the U.S. in Caucasian men > 50 years of age with > 5 years of symptoms. Current recommendations include routine endoscopy and biopsy (looking for dysplastic changes). If two endoscopies and biopsy sessions performed within 12 months are negative for dysplasia then surveillance can be performed every 3 years while the underlying reflux is controlled with proton pump inhibitor drugs in combination with measures to prevent reflux. For patients found to have low grade or high grade dysplasia close observation and repeat endoscopy and biopsies are indicated and the patient should be followed closely by a gastroenterologist.
Proton pump inhibitor drugs have not yet been proven to prevent oesophageal cancer. Laser treatment is used in severe dysplasia, while overt malignancy may require surgery, radiation therapy, or systemic chemotherapy. Additionally, a recent 5-year random-controlled trial has shown that photodynamic therapy using photofrin is statistically more effective in eliminating dysplastic growth areas than sole use of a proton pump inhibitor.[10] There is presently no reliable way to determine which patients with Barrett's oesophagus will go on to develop oesophageal cancer, although a recent study found that the detection of three different genetic abnormalities were associated with as much as a 79% chance of developing cancer in 6 years.[11]
Endoscopic mucosal resection (EMR) has also been evaluated as a management technique.[12] Additionally an operation known as a Nissen fundoplication can reduce the reflux of acid from the stomach into the oesophagus.[13]
In a variety of studies, non-steroidal anti-inflammatory drugs (NSAIDS), like aspirin, have shown evidence of preventing oesophageal cancer in Barrett's oesophagus patients.[14][15] However, none of these studies have been randomized, placebo controlled trials, which are considered the gold standard for evaluating a medical intervention. In addition, the best dose of NSAIDs for cancer prevention is not yet known.
Prognosis
Barrett's oesophagus is only a premalignant condition. Its malignant sequela, oesophageal adenocarcinoma, has a mortality rate of over 85%.[16] The risk of developing oesophageal adenocarcinoma in people who have Barrett's oesophagus is 6-7 per 1000 person-years.[17][18] Most patients with oesophageal carcinoma survive less than 1 year.[19]
Epidemiology
The incidence in the United States among Caucasian men is 8 times the rate among Caucasian women and 5 times greater than African American men. Several studies have estimated the prevalence of Barrett's oesophagus in the normal population to be 1.6%,[20] 1.3%,[21] and 3.6%.[22]
History
Barrett first described the columnar metaplasia in 1950.[4] An association with gastroesophageal reflux was made in 1953.[23] An association with adenocarcinoma was made in 1975.[24]
Additional images
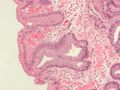 Micrograph of Barrett's oesophagus. Alcian blue stain. |
References
- ↑ Stein H, Siewert J (1993). "Barrett's oesophagus: pathogenesis, epidemiology, functional abnormalities, malignant degeneration, and surgical management". Dysphagia 8 (3): 276–88. doi:10.1007/BF01354551. PMID 8359051.
- ↑ 2.0 2.1 2.2 2.3 2.4 Shaheen NJ, Richter JE (March 2009). "Barrett's oesophagus". Lancet 373 (9666): 850–61. doi:10.1016/S0140-6736(09)60487-6. PMID 19269522.
- ↑ Koppert L, Wijnhoven B, van Dekken H, Tilanus H, Dinjens W (2005). "The molecular biology of oesophageal adenocarcinoma". J Surg Oncol 92 (3): 169–90. doi:10.1002/jso.20359. PMID 16299787.
- ↑ 4.0 4.1 BARRETT NR (October 1950). "Chronic peptic ulcer of the oesophagus and 'oesophagitis'". Br J Surg 38 (150): 175–82. doi:10.1002/bjs.18003815005. PMID 14791960.
- ↑ Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL (August 2007). "Central adiposity and risk of Barrett's oesophagus". Gastroenterology 133 (2): 403–11. doi:10.1053/j.gastro.2007.05.026. PMID 17681161.
- ↑ Reid BJ, Li X, Galipeau PC, Vaughan TL (February 2010). "Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis". Nat. Rev. Cancer 10 (2): 87–101. doi:10.1038/nrc2773. PMID 20094044.
- ↑ Fléjou J (2005). "Barrett's oesophagus: from metaplasia to dysplasia and cancer". Gut 54 Suppl 1: i6–12. doi:10.1136/gut.2004.041525. PMID 15711008.
- ↑ Elizabeth Pohler, Ashley L. Craig, James Cotton, Laura Lawrie, John F. Dillon, Pete Ross, Neil Kernohan and Ted R. Hupp (2004). "The Barrett’s Antigen Anterior Gradient-2 Silences the p53 Transcriptional Response to DNA Damage". Molecular and Cellular Proteomics 3 (6): 534–547. doi:10.1074/mcp.M300089-MCP200. PMID 14967811.
- ↑ Murray E, McKenna EO, Burch LR, Dillon J, Langridge-Smith P, Kolch W, Pitt A, Hupp TR (2007). "Microarray-formatted clinical biomarker assay development using peptide aptamers to anterior gradient-2". Biochemistry 46 (48): 13742–51. doi:10.1021/bi7008739. PMID 17994709.
- ↑ Overholt BF, Wang KK, Burdick JS, et al. (2007). "Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia.". Gastrointestinal endoscopy 66 (3): 460–8. doi:10.1016/j.gie.2006.12.037. PMID 17643436.
- ↑ Galipeau P, Li X, Blount PL, Maley CC, Sanchez CA Odze RD, Ayub K, Rabinovitch PS, Vaughan TV, Reid BJ (2007). "NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to oesophageal adenocarcinoma". PLoS Medicine 4 (2): e67. doi:10.1371/journal.pmed.0040067. PMID 17326708.
- ↑ Reshamwala P, Darwin P (2006). "Endoscopic management of early gastric cancer". Curr Opin Gastroenterol 22 (5): 541–5. doi:10.1097/01.mog.0000239870.04457.80. PMID 16891887.
- ↑ Abbas A, Deschamps C, Cassivi SD, et al. (2004). "The role of laparoscopic fundoplication in Barrett’s oesophagus". Annals of Thoracic Surgery 77 (2): 393–396. doi:10.1016/S0003-4975(03)01352-3. PMID 14759403.
- ↑ Corley DA, Kerlikowske K, Verma R, Buffler P (2003). "Protective association of aspirin/NSAIDs and oesophageal cancer: a systematic review and meta-analysis.". Gastroenterology 124 (1): 47–56. doi:10.1053/gast.2003.50008. PMID 12512029.
- ↑ Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez, CA, Rabinovitch PS, Reid BJ (2005). "Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study". Lancet Oncol 6 (12): 945–52. doi:10.1016/S1470-2045(05)70431-9. PMID 16321762.
- ↑ Holmes RS, Vaughan TL (January 2007). "Epidemiology and pathogenesis of oesophageal cancer". Semin Radiat Oncol 17 (1): 2–9. doi:10.1016/j.semradonc.2006.09.003. PMID 17185192.
- ↑ Thomas T, Abrams KR, De Caestecker JS, Robinson RJ (December 2007). "Meta analysis: Cancer risk in Barrett's oesophagus". Aliment. Pharmacol. Ther. 26 (11-12): 1465–77. doi:10.1111/j.1365-2036.2007.03528.x. PMID 17900269.
- ↑ Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L (August 2008). "The incidence of oesophageal cancer and high-grade dysplasia in Barrett's oesophagus: a systematic review and meta-analysis". Am. J. Epidemiol. 168 (3): 237–49. doi:10.1093/aje/kwn121. PMID 18550563.
- ↑ Polednak AP (May 2003). "Trends in survival for both histologic types of oesophageal cancer in US surveillance, epidemiology and end results areas". Int. J. Cancer 105 (1): 98–100. doi:10.1002/ijc.11029. PMID 12672037.
- ↑ Ronkainen J, Aro P, Storskrubb T, et al. (December 2005). "Prevalence of Barrett's oesophagus in the general population: an endoscopic study". Gastroenterology 129 (6): 1825–31. doi:10.1053/j.gastro.2005.08.053. PMID 16344051.
- ↑ Zagari RM, Fuccio L, Wallander MA, et al. (October 2008). "Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study". Gut 57 (10): 1354–9. doi:10.1136/gut.2007.145177. PMID 18424568.
- ↑ Kim JY, Kim YS, Jung MK, et al. (April 2005). "Prevalence of Barrett's oesophagus in Korea". J. Gastroenterol. Hepatol. 20 (4): 633–6. doi:10.1111/j.1440-1746.2005.03749.x. PMID 15836715.
- ↑ ALLISON PR, JOHNSTONE AS (June 1953). "The oesophagus lined with gastric mucous membrane". Thorax 8 (2): 87–101. doi:10.1136/thx.8.2.87. PMID 13077502.
- ↑ Naef AP, Savary M, Ozzello L (November 1975). "Columnar-lined lower oesophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's oesophagus with 12 adenocarcinomas". J. Thorac. Cardiovasc. Surg. 70 (5): 826–35. PMID 1186274.
External links
- Barrett's oesophagus at National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD)
- Barrett's Info a peer-reviewed web site of information on Barrett's oesophagus and its clinical management.
- Barrett's oesophagus at Johns Hopkins University
- Barrett's oesophagus Video Overview and Barrett's oesophagus Health Information at Mayo Clinic
- Barrett's Oesophagus Campaign Originally The Barrett's Oesophagus Foundation, the UK charity committed to research into prevention of adenocarcinoma of the oesophagus
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||